Species of Thismia may are in my biased opinion the most remarkable and mysterious plants on this planet. But studying them is complicated, as most species are known from only very few collections made in remote rain forest areas. Luckily, there are exceptions. In Australia and New Zealand there are a few species of Thismia, and in recent years more and more localities have been discovered. Nevertheless, even down under Thismia has a very patchy distribution and is considered to be a very rare plant.
Since these plants rely entirely on their mycorrhizal fungi for survival, they are excellent examples to study potential limitations in their distribution due to their interactions. In other words: if their preferred fungus is absent in a region, do they fail to colonize this region, or can they adapt and start exploiting another fungus? That is exactly what I wanted to address in the project ‘Does specialization lead to rarity?’ funded by the Netherlands Organization for Scientific Research (NWO).

Thismia clavarioides (Thismiaceae) – Morton National Park, Australia
First of all, we needed samples. With the help of several local experts we collected specimens of Thismia from as many populations as possible in Australia and New Zealand. This was an unforgettable experience!
We managed to sample many more populations than expected, and during the field work we discovered several new localities and even a putative new species. The DNA of all plant specimens was analyzed to reconstruct their evolutionary relationships. At the same time, we identified the mycorrhizal fungi in the roots of each plants by DNA barcoding. The results look like this:
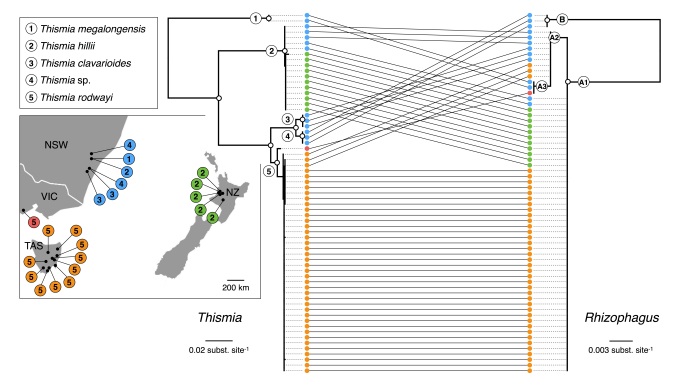
Almost all sampled specimens of Thismia grown with the same fungus (or very narrow lineage of fungi – we don’t really know). Only one species was found growing with an different lineage of fungi. Evolutionary reconstructions indicate that this specificity was already present in the common ancestor of these species, and apparently it did not limit the recent radiation and dispersal (from Australia to Tasmania and New Zealand) of the plants. The fungus these plants use is probably quite widespread, and does not seem to limit the distribution of the plants. Therefore, specialization is not necessarily an evolutionary disadvantage (as it is sometime seen), particularly if your host is successful and widespread.
The full paper published in Journal of Biogeography can be found here (open access): http://onlinelibrary.wiley.com/doi/10.1111/jbi.12994/full